What is the bond order of the carbon- carbon bond in the acetaldehyde molecule. Draw the Lewis structure for chloroacetylene C2HCI.

Draw A Lewis Structure Of Formaldehyde Lewis College Life Hacks Middle School Science
A compound with a molar mass of about 28 gmol contains 857 carbon and 143 hydrogen by mass.

. It was formed in the kidneys because only S-12-dichlorovinyl glutathione was found in the bile. Lewis Structures of Acetaldehyde. Enter a number Which species has the shortest carbon.
Ethanal consisting of an aldehyde functional group comprises two Carbon atoms with an Oxygen atom double-bonded to the rmC 1 Carbon atom. Since Hydrogen is in Group I it has one 1 valence electron in its shell. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs.
Here in C-H bond the difference. Each atom is thus neutral. We review their content and use your feedback to keep the quality high.
First determine the total number of valence electrons in the molecule. Excerpt from ERG Guide 130P Flammable Liquids Water-Immiscible Noxious. Around iodine there are 7 valence electrons one of which is involved in the C I bond.
The exception of course being the hydrogens. It is derived from acetic acid. 344 255 089.
It is used as a plasticizer hygroscopic agent and fire suppressant. Write the Lewis structure for a molecule of. Acetamide is the simplest amide.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. A difference in electronegativity among two atomic elements in the range of around 04 2 is considered to be polar covalent bonds. As an immediate precautionary measure isolate spill or leak area for at least 50 meters 150 feet in all directionsLARGE SPILL.
No symbol for that. Acetamide CH3CONH2- Acetamide is a chemical compound having the formula CH3CONH2. Draw The Lewis Structure For Chloroacetylene.
How to Draw the Lewis Dot Structure for C2H3Cl. It can also be obtained through ammonolysis of acetylacetone with the under conditions that are used in reductive amination. Biliary cannulation did not influence.
If tank rail car or tank truck is involved in a fire ISOLATE for 800 meters 12 mile in all. Metabolites in the urine were identified as N-acetyl-S-12-dichlorovinyl-L-cysteine dichloroethanol dichloroacetic acid oxalic acid and chloracetic acid after inhalation exposure of rats. Consider initial downwind evacuation for at least 300 meters 1000 feet.
Draw the Lewis Dot Structure for the Hydrogen atom. Chloroethylene Vinyl chlorideFor the C2H3Cl structure use the periodic table to find the total number of va. Visit BYJUS for more content.
The hydrogen has one cross shared with one dot from the first carbon. Only the cysteine conjugate was found in feces. H-CC-Cl is Ethylene Chloride There is a triple bond between the carbons.
They follow the duet rule 2 electrons. In the Lewis structure for Acetaldehyde there are a total of 18 valence electrons. A hydrogen atom has a valency of one as it only one electron in its outer shell.
Here we have a diagram of Pauling electronegativity chart. This will be the sum of the group number a of all atoms plus the charge. Ethanal is most likely a carcinogen in humans.
Around each carbon there are 6 electrons 4 of which are involved in covalent bonds. Ć C - o X x s. In C and O bond the difference.
Try reading the explanation first then. Experts are tested by Chegg as specialists in their subject area. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3 satisfying the octet rule but experimental evidence indicates the bond lengths are closer to that expected for BF single bonds.
70 More Lewis Dot Structures. Be certain you include any lone pairs. Draw Lewis structures for the acetaldehyde molecule CH 3 CHO the fluoroethene molecule C 2 H 3 F and the chloroethyne molecule C 2 HCl in the window below and then answer the questions that follow based on your drawings.
255 220 035. Lewis Dot of Acetaldehyde ethanal CH 3 CHO. View Notes - Drawing Lewis Dot Structure from CHEM 142 at University of Washington Tacoma.
The following is an example of how to draw the best Lewis structure for NO 3-learning by example. 100 13 ratings Transcribed image text. Around each hydrogen there is 1 electron.
A the amino acid serine. Learn more about Acetamide structure Properties Production and Uses with Frequently asked Questions here. ALEKS 101116 644 PM Explanation Incorrect.
This suggests the best Lewis structure has three BF single bonds and an electron deficient boron. Do not add any more atoms. Who are the experts.
O x 3 18. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. These electrons are distributed as shown below.
This type of Lewis dot structure is represented by an atomic symbol and a series of dots.

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Exp Chemistry Classroom Teaching Chemistry Chemistry Lessons
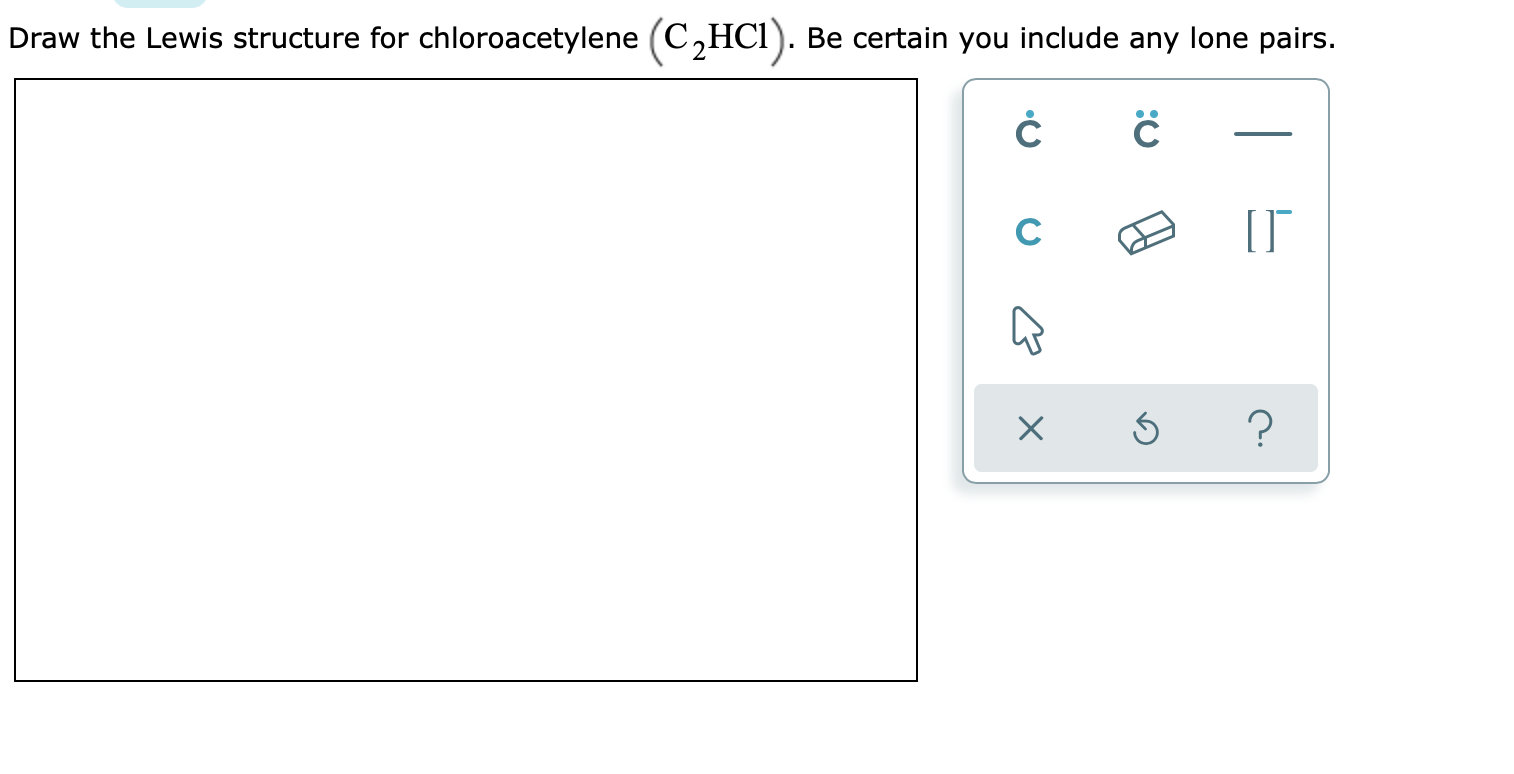
Solved Draw The Lewis Structure For Chloroacetylene C2hci Chegg Com

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo Teaching Science High School Chemistry Organic Chemistry Study
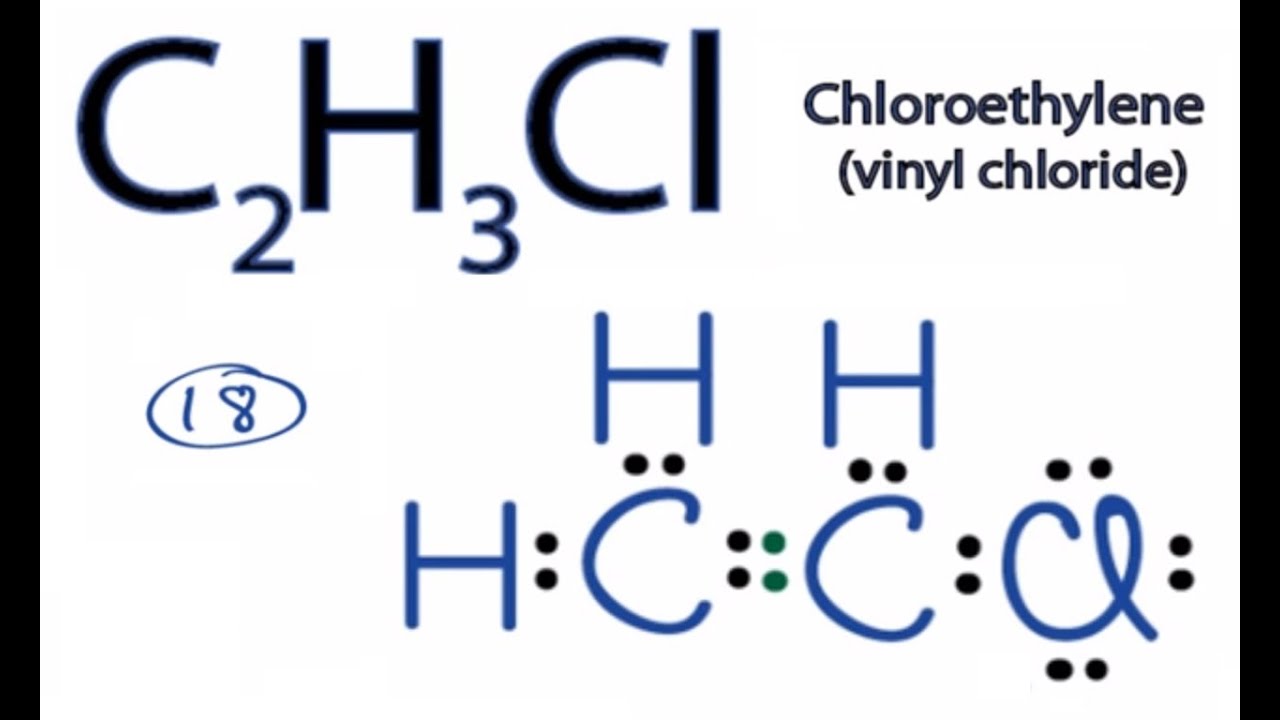
C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

Cs2 Lewis Structure Chemistry Worksheets Lewis Electron Configuration

Drawing Lewis Structures Resonance Structures Chemistry Tutorial Youtube Chemistry Organic Chemistry Science Education

Ch4o Lewis Structure How To Draw The Lewis Structure For Ch4o Youtube
0 comments
Post a Comment